
Approximately 157,000 are Affected need to be examined
The manufacturer, Medtronic must call back 157.000 his pacemaker. The reason for this is a possible device failure. For all Concerned, even additional talk to be given to hours in the heart centers. The Federal Agency for medicines and medical devices recently published a communication with all the important information for those Affected.
The largest recall action in the field of cardiology
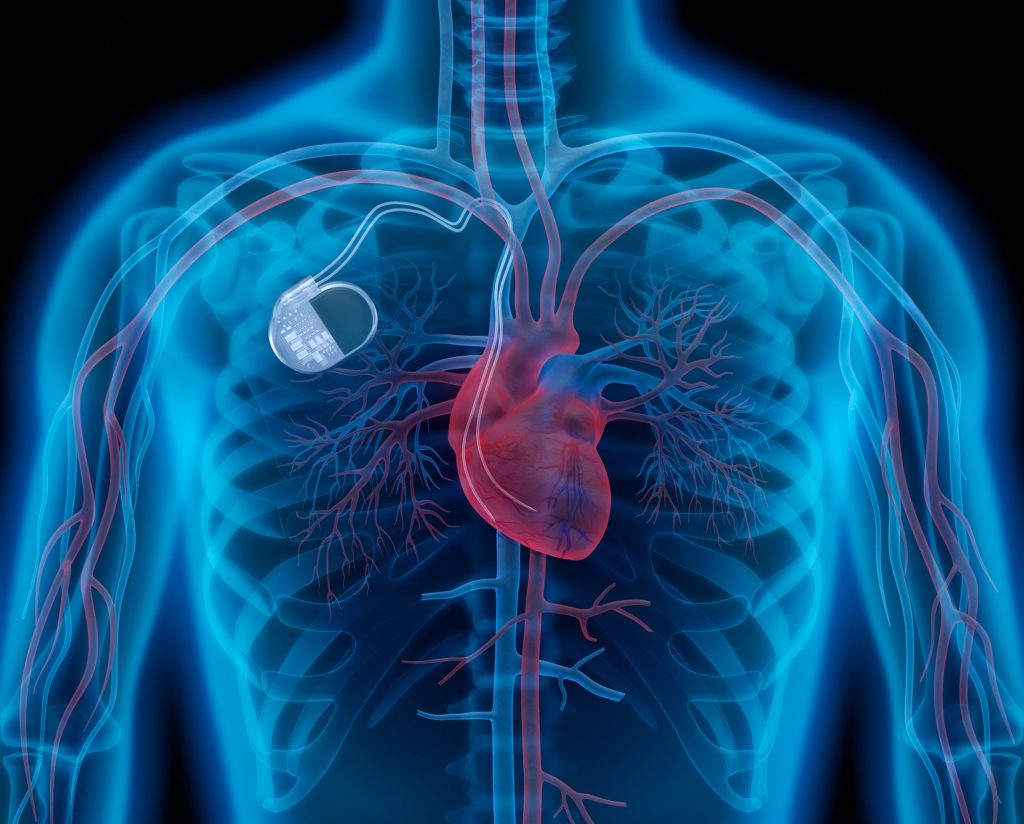
The problem lay most probably in the programming of the device, explains Professor Andreas Franke from the Siloah hospital, Klinikum Region Hannover to the NDR. The result is that the pacemaker will interpret in rare cases, the cardiac impulses are wrong and will not work. For most patients, this is probably not a Problem, Franke. But as a precaution, all patients with these special pacemakers to the manufacturer, Medtronic would have to be controlled. The chief physician speaks of the largest recall action in the field of cardiology. It is assumed that all heart centers in Germany and have used this error pacemakers in patients.
Operations are only in a few cases, necessary
Alone in the Klinikum Region Hannover must be controlled according to the NDR now almost 600 patients with relevant cardiac pacemakers. In the five hospitals of the Hannover Region special office hours were set up so that the nearly 600 patients affected can be advised. Chief physician, Franke expects that in most cases, a Software Update will be sufficient, in order for the device to work properly again. Only in a few cases, surgery was required.
Manufacturer Medtronic hopes to have an Update in the second half of the year
What time is the Update from the manufacturer comes, not fixed yet. The medical devices group Medtronic hopes to be able to this Update in the second half of the year deliver. The safety information published by the Federal Institute for drugs and medical products.
These types of pacemakers are affected
The affected pacemakers may interpret cardiac impulses is wrong. A Software Update should fix the Problem. The security information shows that a total of seven different types of pacemakers are affected. The brand name of the Medtronic pacemakers are:
- Adapta,
- Versa,
- Sensia,
- Relia,
- SOA IC.,
- Sphera,
- and Vitatron.
Patients and Physicians can determine whether a specific device is affected by entering the serial number on the Product Performance page of Medtronic. A Link to this page, you can find here.
The chamber of Physicians calls for a national Register for implants
How many patients must now nationwide examine, is not fixed yet. Medtronic has made to date. In addition, there is no national Register for implants. Such a directory would finally come, the lower Saxony chamber of Physicians. “We also need a government-approved certificate for Implantate“, spokesman Thomas Spieker stressed to NDR. So far, the manufacturer could choose their appraiser throughout Europe. (fm)