
Considering a patient’s genetics could inform clinicians which medications would be most effective in controlling cravings and treating alcohol use disorder.
Twenty million Americans currently struggle with an alcohol use disorder. Of those who seek treatment, only 20% receive medications, either alone or in addition to counseling.
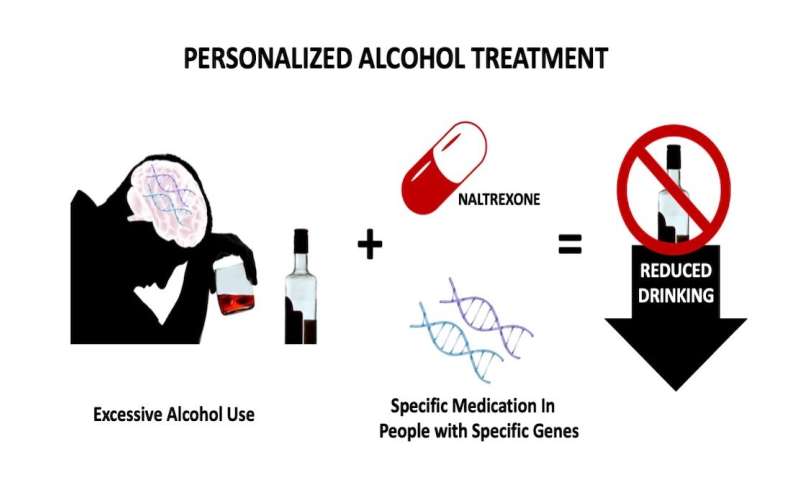
Medications are not used more often, according to Charleston Alcohol Research Center scientific director Raymond Anton, M.D., in part because they do not work equally well for everyone. Many patients with alcohol use disorder would benefit from a personalized medicine approach, in which a medication is prescribed based on a patient’s genetic code.
Anton and his team report in Alcoholism: Clinical and Experimental Research that doing a few relatively simple genetic tests to identify variations in just three brain genes makes it possible to predict which patients with an alcohol use disorder will benefit most from the addiction treatment medication naltrexone.
In previous studies, Anton’s team showed that treating alcohol use disorder with medications that work on specific brain chemicals can reduce the relapse rate by up to a third.
“Alcohol dependence is a brain disease known to affect certain brain chemicals,” said Anton, “So, it’s important to use treatment methods that address not only the behavioral but also the biological/brain components of the problem.”
Naltrexone, a Food and Drug Administration (FDA)-approved addiction medication, is somewhat unique in that it targets just a single protein in the brain -the mu-opioid receptor. When activated by either an internally produced or externally introduced opioid-like chemical, the mu-opioid receptor signals a positive experience. Drinking alcohol releases natural opiates in the brain that activate the mu-opioid receptor. Naltrexone blocks the mu-opioid receptor to prevent the reward and pleasure that comes from drinking alcohol and can even reduce the craving to consume it.
The gene that produces the mu-opioid receptor protein in the brain is not the same in every patient. In the current study, Anton and his team considered the influence of a small gene variation that results in a slight difference in the mu-opioid receptor protein structure.
That slight difference does not affect how people act under normal situations, but it does cause a subtle difference in how strongly the mu-opioid receptor becomes activated when alcohol is consumed, with one variation having a greater response than the other.
Anton and his team hypothesized that this subtle difference in brain chemistry might affect how well naltrexone works in any given patient.
They quickly discovered, however, that the variation in this one gene only did not fully predict how well a patient would respond to the medication.
“There is a small indication that the difference in the mu-opioid receptor gene sequence matters, but it isn’t a powerful predictor,” Anton explained. “People are far more complex than one individual gene variation. Naltrexone targets this specific mu-opioid receptor, so we hypothesized that the other brain chemicals that might influence the mu-opioid receptor could also influence how the drug might work.”
Dopamine is another reward and pleasure signaling system in the brain that often interacts with the opioid system. Therefore, the amount of dopamine present could influence the mu-opioid receptor and thus the effectiveness of naltrexone.
Anton and his team looked at two such genes that produce proteins controlling the amount of dopamine in the brain.
Like the mu-opioid receptor, these dopamine-processing genes can have small specific variations that result in slight differences in the strength of reward or pleasure signaling after alcohol consumption.
In a clinical trial, Anton and his team genotyped 146 treatment-seeking alcohol use disorder patients for the selected variations in the mu-opioid receptor gene and the two dopamine-processing genes. A roughly equal number of patients with each gene variation were assigned randomly to receive naltrexone or an identical-looking placebo medication.
Throughout the 16-week trial funded by the National Institutes of Health, patients reported how much they drank each day. A reduction in the number of binge-drinking days, defined as five or more drinks for men or four or more drinks for women, across the study indicated a positive effect of the medication.
Anton and his team found that only patients with certain combinations of gene variations showed consistently reduced drinking when taking naltrexone.
“To benefit most from naltrexone, you have to have the gene variations that predict you’ll be low in one brain chemical response -dopamine or mu-opioid -and high in the other,” Anton explained.
This finding indicates that patients can be genotyped before treatment to see if they will benefit from naltrexone. If they will not benefit, other medications that might be effective are available for them.
Source: Read Full Article