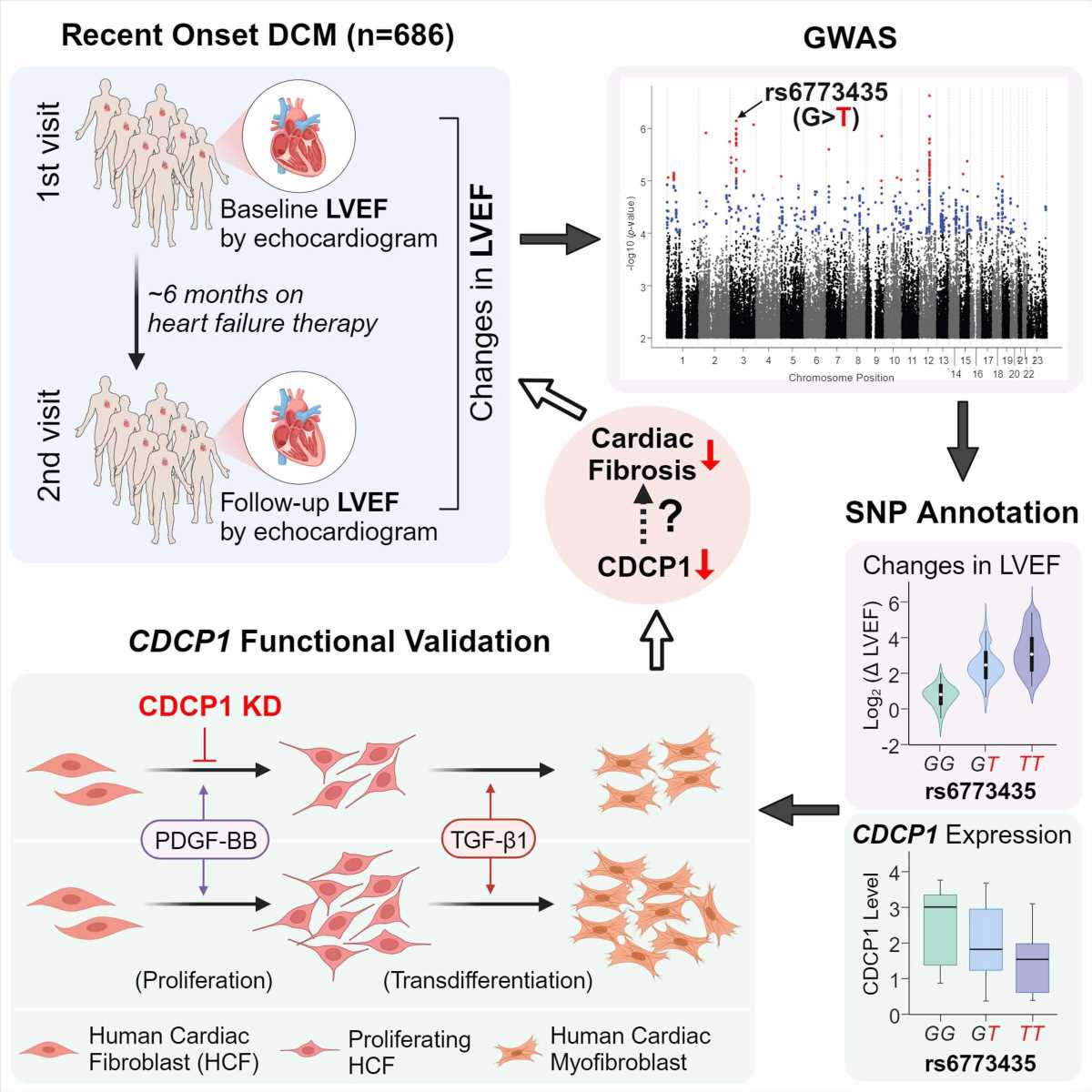
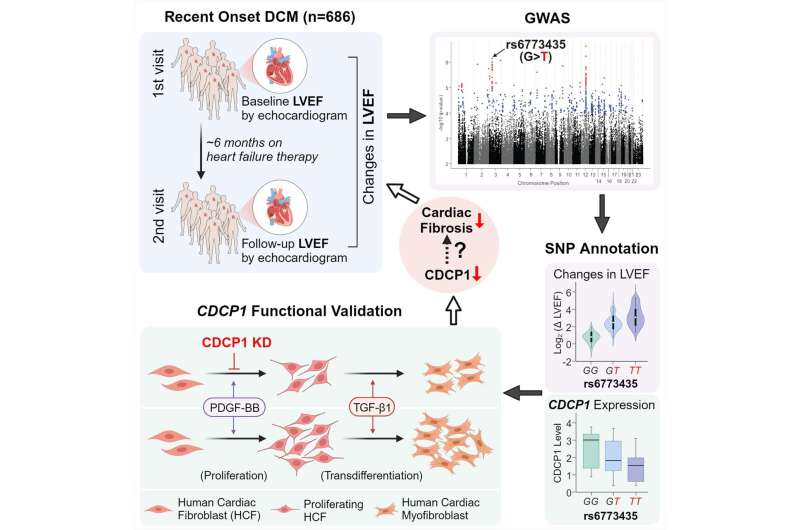
Mayo Clinic researchers studying the genetics of people who had recently developed dilated cardiomyopathy, one of the most common causes of heart failure, have found a particular gene to target for developing future drug therapy treatments. The disease makes it harder for the heart’s left ventricle to pump blood effectively to the rest of the body.
In this first genome-wide association study, now published in Circulation Research, the researchers sought to understand why some patients get better after developing the condition—and some don’t.
“We found genetic variation in the CDCP1 gene, a gene that no one has heard of in cardiology, and its link to improvement in heart function in these patients,” says lead author Naveen Pereira, M.D., a Mayo Clinic cardiologist, who studies genetic variation and its implications in diagnosing and treating cardiovascular disease, specifically heart failure.
Genetic variation in the CDCP1 gene can lead to differences in the protein’s structure, potentially influencing a person’s susceptibility to various diseases or their response to specific therapies.
The researchers identified and examined the role of the CDCP1 gene because of this gene’s link to improving the heart’s left ventricle to pump blood effectively in people with dilated cardiomyopathy. The CDCP1 gene is often variably expressed in fibroblasts (connective tissue) of people with this condition. In addition, fibrosis (excess fibrous connective tissue in the heart) plays an essential role in the prognosis of this disease.
Dr. Pereira notes interestingly that they also found that genetic variation in or near CDCP1 was significantly associated with death due to heart failure.
They also noted that decreasing this gene’s expression in cardiac connective tissue significantly decreased cardiac fibroblast proliferation and downregulated the IL1RL1 gene. This gene encodes one of the most important heart failure biomarkers, sST2. High levels of this biomarker are associated with fibrosis and death; a decrease in CDCP1 decreases the expression of this protein. Understanding the regulation of sST2 and its relationship with CDCP1 and fibrosis is essential for developing strategies to mitigate the adverse effects of heart failure.
Dr. Pereira explains that these findings raise the possibility of targeting the CDCP1 gene to decrease cardiac fibrosis, which may improve heart function. The study, therefore, has implications for developing new drug therapies for dilated cardiomyopathy and potentially other conditions affected by fibrosis.
According to a report from the American Heart Association, heart failure is an increasingly common diagnosis in the U.S., with a projection of affecting more than 8 million people by 2030, an increase of 46% from the present day.
Between 30% and 40% of heart failure cases are due to dilated cardiomyopathy.
“It is the most common cause of a person needing a heart transplant,” says Dr. Pereira. “A key indicator of whether patients with dilated cardiomyopathy will recover is whether they have cardiac fibrosis.”
Based on these preliminary findings, the Mayo researchers are doing further animal studies to understand the effect of CDCP1 on heart failure. They are developing molecules to assess their therapeutic potential for dilated cardiomyopathy and heart failure.
“By continuing with this research that started with a human population that we took to the molecular and now animal laboratory, we hope to find new avenues for treatments to take back to the human population we studied, to improve patients’ survival and quality of life ultimately,” says Dr. Pereira.
More information:
Duan Liu et al, Myocardial Recovery in Recent Onset Dilated Cardiomyopathy: Role of CDCP1 and Cardiac Fibrosis, Circulation Research (2023). DOI: 10.1161/CIRCRESAHA.123.323200
Journal information:
Circulation Research
Source: Read Full Article