Anorexia nervosa (AN) is the deadliest psychiatric illness aside from opioid use disorder, with scarce effective treatment options. A new study by Yale researchers reveals a potential new therapeutic for the disorder, as well as for cancer-induced anorexia and other mood disorders.
While scientists still don’t understand the molecular mechanisms that underlie anorexia, a team led by principal investigator Yingqun Huang, MD, Ph.D., professor of obstetrics, gynecology & reproductive sciences at Yale School of Medicine, found that a small synthetic molecule called Bobcat339 significantly weakened AN, as well as associated anxiety and depressive-like behaviors, in a mouse model, causing few side effects.
The team hopes the findings, published in Proceedings of the National Academy of Sciences, is a step toward developing new and safe therapeutics.
“This small molecule, Bobcat339, can mitigate AN in a mouse model,” says Huang. “So, we propose that it may also be effective in treating human AN and perhaps cancer-induced anorexia and associated mood disorders.”
A surprise in the lab points researchers toward anorexia
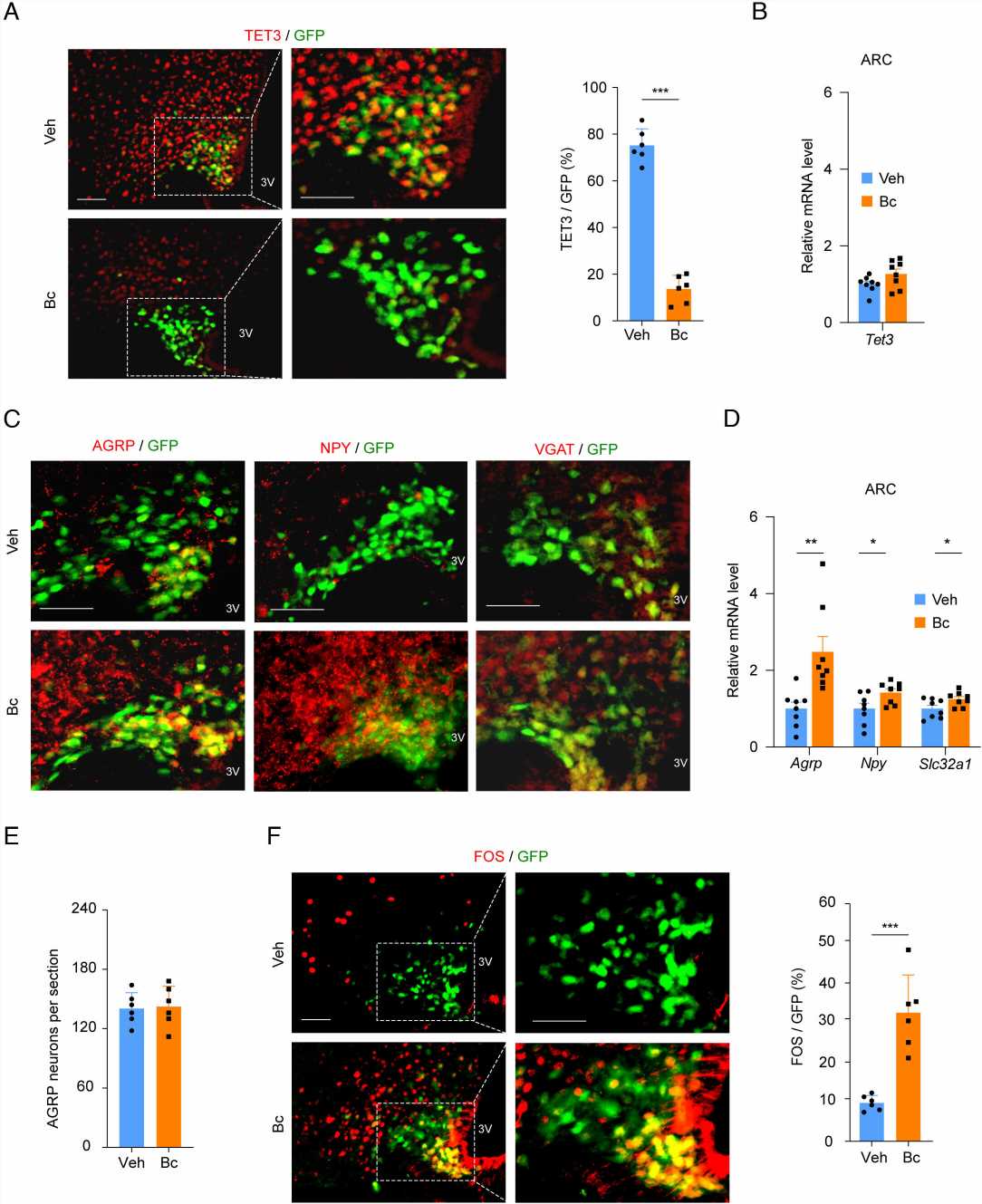
The discovery of Bobcat339, says Huang, was “a serendipitous thing.” She had been studying a protein called TET3, a member of the TET protein family (TET1/2/3), in the context of type 2 diabetes and found in 2020 that its expression was increased in the livers of patients with diabetes. Through delivering genetic material known as small interfering RNAs (siRNAs) that targeted TET3 specifically to the livers of mice, her team discovered that they could attenuate type 2 diabetes in animal models.
Then, Huang learned that Bobcat339 is a TET protein inhibitor. Intrigued, she hoped it might be a good molecule for treating type 2 diabetes. To her surprise, after ingesting Bobcat339 through their drinking water, the mice did not get better. “The mice began eating like crazy—hyperphagia,” says Huang. “At first, this was so disappointing.”
But the path that Bobcat339 took through the mice’s bodies led to a crucial finding. After Huang’s team fed the mice the molecule, they discovered that it also traveled to the brain, where it regulated TET3 by interacting with AgRP neurons. This group of neurons, located in the hypothalamus, plays a significant role in regulating feeding, energy expenditure, and other complex behavior including anxiety, depression, and compulsive behaviors.
“These neurons are very important not only for regulating food intake, but also mood,” says paper co-author Tamas Horvath, DVM, Ph.D., chair and Jean and David W. Wallace Professor of Comparative Medicine and professor of neuroscience and of obstetrics, gynecology & reproductive science.
This led to another publication, in the Journal of Clinical Investigation, in which Huang and Horvath teams used CRISPR knockdown of TET3 specifically in AgRP neurons in mice and discovered this induced hyperphagia as well as anti-anxiety and anti-depressive effects. This sparked the teams’ interest in using Bobcat339 to knockdown TET3 and treat an entirely different condition altogether—AN.
Anorexia damages the body and mind
AN is characterized by reduced food intake, low body weight, and obsessive-compulsive activity like overexercising, as well as mood disorders such as depression and anxiety. It is notoriously difficult to treat. Even after patients recover in terms of their food intake, body weight, and exercise level, mood problems often persist. The condition can be treated with psychotherapy and nutritional support, but these interventions have low efficacy and high relapse rates. There is no medication available for AN.
The activity-based anorexia model is a well-established model in which mice are kept in an apparatus with a running wheel. By withholding food from mice and only making it available for two hours a day for three days, researchers can induce anorexia behavior. The mice quickly reduce their food intake, lose body weight, and exhibit obsessive-compulsive wheel-running behavior. After three days, the researchers resume normal feeding.
Huang’s team took measurements during both the restrictive and recovery periods. Mice that were administered Bobcat339 through intraperitoneal injections showed significantly higher food intake and lower compulsive wheel running than untreated mice during the restrictive period. Upon resuming normal feeding, although mice in the untreated control group regained their body weight, they still exhibited depressive and anxious behaviors.
“In humans, AN usually manifests during puberty, but mood problems often persist through adulthood. A similar thing happened with our mouse model,” says Huang.
In contrast, mice treated with Bobcat339 showed fewer of these behaviors. “This small molecule helps mice maintain their food intake, maintain their body weight, and inhibit compulsive behaviors,” she says. “This drug is also working in mitigating anxious and depressive-like behaviors.”
The team hopes that the molecule may also be useful in treating humans struggling with anorexia nervosa, as well as those struggling with mood disorders. In contrast to typical anti-depressants, which can need weeks to take effect, Bobcat339 was found to improve problems associated with mood in as little as a day. The team also plans to conduct future studies evaluating its ability to mitigate cancer-induced anorexia and depression.
More information:
Haining Lv et al, A small-molecule degrader of TET3 as treatment for anorexia nervosa in an animal model, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2300015120
Di Xie et al, TET3 epigenetically controls feeding and stress response behaviors via AGRP neurons, Journal of Clinical Investigation (2022). DOI: 10.1172/JCI162365
Journal information:
Proceedings of the National Academy of Sciences
,
Journal of Clinical Investigation
Source: Read Full Article