
Alzheimer’s disease is the most prominent cause of dementia affecting millions of people worldwide. As the changes in the brain function starts 10–20 years before the clinical onset of Alzheimer’s disease, there is a strong interest in the identification of early markers that can be predictive of future mental health/cognitive decline. This is something that we researchers at the Karolinska Institute are on the lookout for. Our latest study is now published in Nature Reviews Neurology.
In this context, astrocytes are one of the promising targets due to their early and swift response to Alzheimer’s disease progression. Astrocytes represent important homeostatic cells in the brain controlling a wide array of functions needed for an optimal brain functioning and homeostasis. Most importantly, they respond to brain insults/injuries and disease state by a specific defense process, reactive astrogliosis.
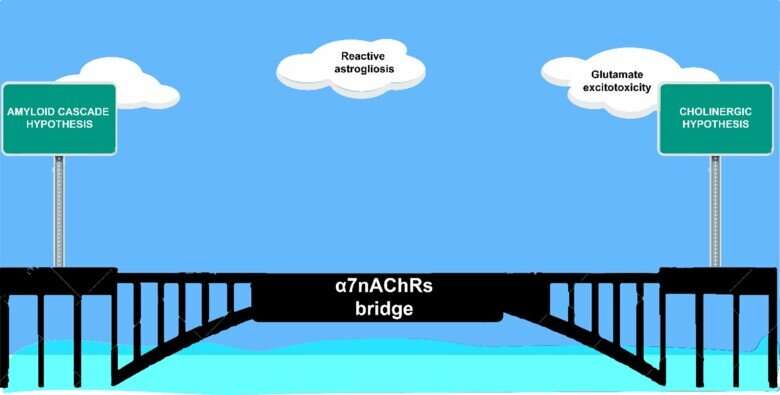
In Alzheimer’s disease, the role of astrocytes is still unclear, and several recent studies show that reactive astrogliosis can precede other well-known pathological hallmarks of Alzheimer’s disease, such as amyloid deposition (Aβ) and tau tangles. It is thus critical to define new astrocytic biomarkers to deepen our understanding of reactive astrogliosis in the Alzheimer’s disease continuum. In this context, the crosstalk between cholinergic signaling and reactive astrogliosis could hold the key for understanding the early responses of glial cells to brain pathology and injury. The landmark cholinergic hypothesis of Alzheimer’s disease that was proposed almost four decades ago paved the way for the development of cholinesterase inhibitors—so far, the gold-standard therapy for Alzheimer’s disease.
In this paper, we revisited the cholinergic signaling pathways with the aim of exploring the last two decades of research on astrocytic α7-subunit of the nicotinic acetylcholine receptors (α7nAChRs) to shed light on their role in the context of Alzheimer’s disease pathology and biomarkers. We discussed the probable involvement of astrocytic α7nAChRs in the instigation and potentiation of early Aβ pathology and highlighted several new mechanistic pathways of AD pathogenesis.
Based on these mechanistic pathways, we proposed that astrocytic α7nAChRs could be an important bridge linking reactive astrogliosis, cholinergic, and the amyloid cascade hypotheses in Alzheimer’s disease. Moreover, targeting astrocytic α7nAChRs as a novel early biomarker with different imaging PET-tracers could be a game changer in the clinical setting for future Alzheimer’s disease diagnostic and therapeutic interventions (see our previous research on inflammatory changes in the brain twenty years before Alzheimer onset and possible new PET tracer for early detection of Alzheimer’s).
We believe this paper will open new avenues in terms of identifying novel biomarkers for early diagnosis of Alzheimer’s disease and new targets for disease-modifying treatments and will have broad clinical implications, encompassing other neurodegenerative disorders in which reactive astrogliosis is also observed. We have already started to put this new hypothesis to the test with our novel in house discovered and developed α7nAChRs PET-tracer KIn-83, which has been extensively characterized in postmortem human brains and soon will reach the first in man PET studies.
More information:
Igor C. Fontana et al, The role of astrocytic α7 nicotinic acetylcholine receptors in Alzheimer disease, Nature Reviews Neurology (2023). DOI: 10.1038/s41582-023-00792-4
Journal information:
Nature Reviews Neurology
Source: Read Full Article