
National Institutes of Health researchers compared a new genetic animal model of Down syndrome to the standard model and found the updated version to be enhanced. The new mouse model shows milder cognitive traits compared to a previously studied Down syndrome mouse model. The results of this study, published in Biological Psychiatry, may help researchers develop more precise treatments to improve cognition in people with Down syndrome.
Scientists found that the new mouse model, known as Ts66Yah, had memory difficulties and behavior traits, but the symptoms were not as severe as seen with the previous mouse model. Scientists often use different strains of mice as animal models to study human diseases because most genes in humans have similar counterparts in mice.
“A mouse model that more precisely captures the genetics of Down syndrome has important implications for human clinical trials that aim to improve cognition,” said Diana W. Bianchi, M.D., director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, senior investigator in the National Human Genome Research Institute (NHGRI) Center for Precision Health Research and senior author of the study.
About 6,000 newborns are diagnosed with Down syndrome each year in the United States. In most cases, these babies have a third copy of chromosome 21. An additional chromosome 21 adds an extra copy of over 200 protein-coding genes to that person’s genome, which causes difficulties with learning, speech and motor skills.
A previous mouse model, known as Ts65Dn, has been considered the standard for Down syndrome research, used in preclinical studies for nearly 30 years. Along with some successful cognitive treatments, such as a recent hormone-based cognitive treatment, some other treatments that were effective in the mouse model were not as effective in humans.
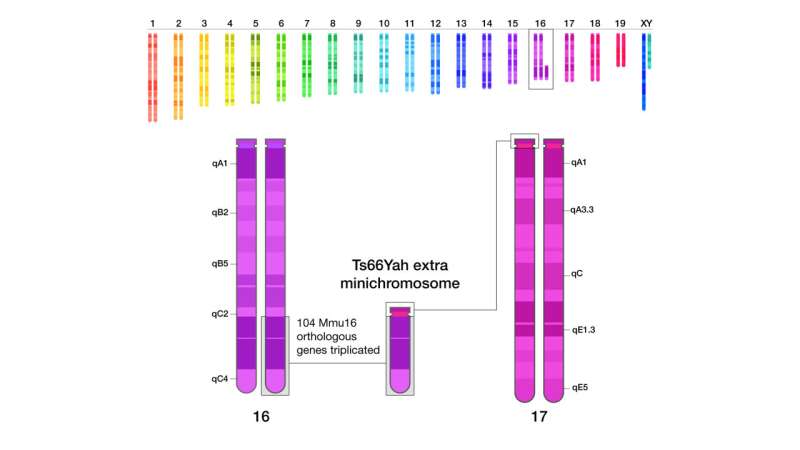
Importantly, the previous mouse model’s genome contains 45 extra genes that are irrelevant to human Down syndrome, a byproduct of how the model was developed. Humans and mice have very similar genomes, but the chromosomes that make up those genomes do not precisely align across those two species.
For example, many of the genes found on human chromosome 21 are found on mouse chromosomes 16 and 17. The previous mouse model has an additional region of mouse chromosome 17 that contains 45 extra genes not found on human chromosome 21. How these 45 extra genes affect the brain and behavior of the previous Ts65Dn mice has not been investigated until now.
To create an enhanced mouse model of Down syndrome, researchers at the University of Strasbourg, France, removed these extra 45 genes using CRISPR gene-editing technology. Dr. Bianchi’s group then compared the two mouse models and found that the extra 45 genes in the previous mouse model were affecting brain development and contributed to more severe difficulties with motor skills, communication and memory.
“There are considerable effects of these extra genes on mouse brain development and behavior,” said Faycal Guedj, Ph.D., staff scientist in NHGRI’s Center for Precision Health Research and first author of the study. “What was previously thought as the best mouse model of Down syndrome has traits derived from genes that are not relevant to human chromosome 21.”
Researchers at the Center for Precision Health Research aim to use cutting-edge genomics tools to foster next-generation healthcare. With this new and improved mouse model, Dr. Bianchi’s group hopes to develop more precise treatments for improving cognition in people with Down syndrome.
“The possibility of treating intellectual disabilities in the context of Down syndrome goes to the core of changing conceptions about the nature of disability, its medical and clinical aspects, and what we, often pejoratively, consider ‘normal’ and ‘desirable’ in the context of medical care and in society,” underscores Christopher R. Donohue, Ph.D., NHGRI senior historian.
“As cognitive treatments based on genetic models become more feasible in the future, researchers, in conversation with disability ethicists, those with Down syndrome and other healthcare professionals, should carefully weigh potential benefits versus drawbacks, including contributing to ableism in medicine, and other forms of stigma.”
More information:
Faycal Guedj et al, The impact of Mmu17 non-Hsa21 orthologous genes in the Ts65Dn mouse model of Down syndrome: the “gold standard” refuted, Biological Psychiatry (2023). DOI: 10.1016/j.biopsych.2023.02.012
Journal information:
Biological Psychiatry
Source: Read Full Article