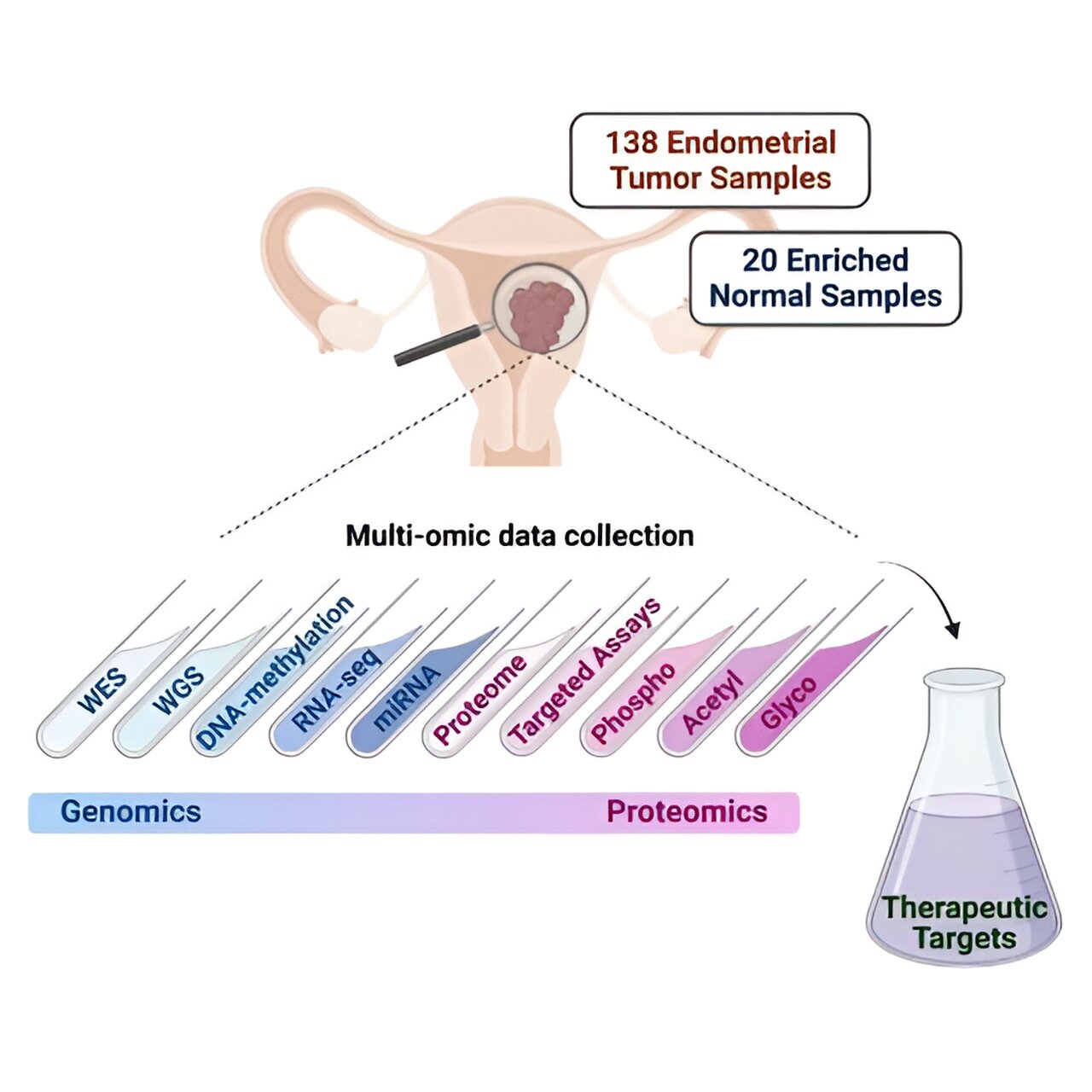
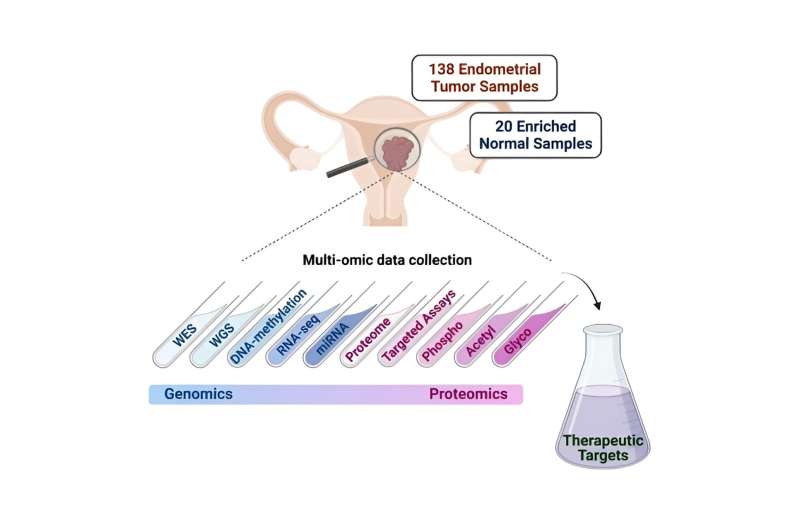
Endometrial carcinoma, a cancer of the lining of the uterus, is the most common gynecologic malignancy in developed nations. Over the past decade, its incidence has steadily increased (approximately 1% annually) and mortality has progressively worsened.
“Despite many attempts to improve this cancer’s treatment, significant challenges remain. In this study, we focused on gaining new insights to improve treatment of endometrial cancer,” said co-first author Dr. Yongchao Dou, postdoctoral associate in Dr. Bing Zhang’s lab at Baylor College of Medicine’s Lester and Sue Smith Breast Center. “We used 10 genomics and proteomics platforms to identify biological markers and pathways that could be used to develop improved therapies for this condition.”
The study appears in the journal Cancer Cell.
Previous genomic studies have depicted the mutational landscape of endometrial cancer. However, how mutations drive cancer characteristics have remained elusive. In 2020, Zhang and colleagues in the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) published the first integrative proteogenomic characterization of endometrial cancer in the journal Cell. They revealed the impact of genetic aberrations on proteins and their chemical modifications, such as phosphorylation, leading to a more comprehensive molecular understanding of this cancer.
“Based on the 2020 study, we propose that not-yet identified molecules involved in endometrial carcinoma progression could be effective targets to control or stop tumor growth,” said co-corresponding author Zhang, professor of molecular and human genetics, the Lester and Sue Smith Breast Center and a McNair Scholar at Baylor. He also is a member of the Dan L Duncan Comprehensive Cancer Center.
A comprehensive investigation
To identify potential new endometrial carcinoma targets, the team conducted a comprehensive proteogenomic investigation—the identification of both genetic components and proteins produced by cancer cells—of 138 prospectively collected endometrial carcinoma tumors and 20 normal endometrium samples.
First, the findings provided functional validation of previously described effects of frequently observed gene mutations, protein markers of clinical and genomic tumor subgroups and the impact of defects in antigen presentation—an important step in triggering an immune response—on patterns of immune infiltrates in some tumors.
“Importantly, our current study also expands our previous work in endometrial cancer in several ways, including the exploration of additional protein types such as glycoproteins, contributing further insights into endometrial cancer biology,” Dou said. “We also developed targeted mass spectrometry-based assays, which provide a path to the development of clinical assays.”
Highlighted findings
The researchers explored in detail the role of mutations called in-frame indels in the PIK3R1-AKT pathway that have been associated with worse cancer outcomes. The researchers hypothesized that in-frame indels eliminate PIK3R1’s ability to suppress cancer progression. PIK3R1 is thought to suppress cancer progression by preventing AKT1 phosphorylation, the addition of a phosphate chemical group to the enzyme AKT1. Phosphorylated AKT1 is thought to activate pathways that help cancer grow.
To gain more evidence supporting their observation of the potential role PIK3R1 proteins with in-frame indels in promoting AKT phosphorylation, the Zhang lab collaborated with Dr. Yi Li, professor of molecular and cellular biology and of molecular virology and microbiology at Baylor. He also is member of Baylor’s Lester and Sue Smith Breast Center and the Dan L Duncan Comprehensive Cancer Center.
“We used CRISPR to engineer in cell lines a specific in-frame indel mutation that is present in a patient’s endometrial cancer cells,” Li said. “Then, the cancer cell line became exactly as the Zhang lab had predicted according to their proteogenomic analysis of cancer samples from patients. We saw tremendous activation of protein AKT, which supported the hypothesis that in-frame indels in PIK3R1 promote AKT phosphorylation. Further studies are needed to evaluate the importance of this mutation in activating AKT leading to cancer growth in living animals, and importantly, whether the tumors will become responsive to the drugs the Zhang group found to be effective for certain patients.”
“Altogether, our findings suggest that PIK3R1 in-frame indel mutations are potential markers of the AKT inhibition response and might be used to identify patients who could respond to AKT inhibitors, moving a step toward future improved clinical applications,” Zhang said. “The idea of this study is to reveal biomarkers that can identify patients who would respond better to treatment. In this large observational study, we have generated a list of potential therapeutic targets for the scientific community to test for their potential clinical value.”
More information:
Yongchao Dou et al, Proteogenomic insights suggest druggable pathways in endometrial carcinoma, Cancer Cell (2023). DOI: 10.1016/j.ccell.2023.07.007
Journal information:
Cancer Cell
,
Cell
Source: Read Full Article