A study by the University of Seville, CABIMER and IRB Barcelona led by the researchers Andrés Aguilera (US-CABIMER) and Aleix Bayona Feliu (IRB Barcelona) raises the possibility that DNA–RNA hybrids and the mutations in various cellular factors that form them may be responsible for the emergence of carcinogenic processes. The authors therefore stress the need to study these factors to understand and assess possible cancer risks.
The paper was published in the journal Nature Communications.
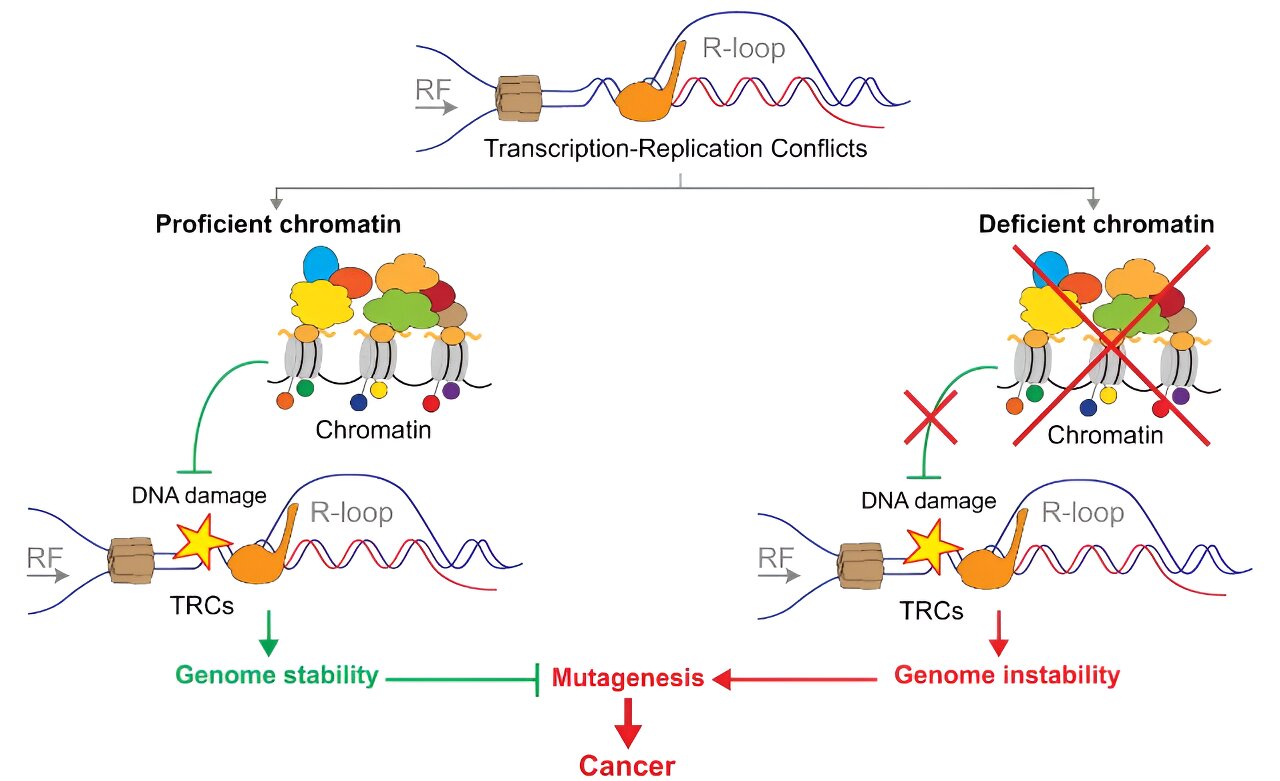
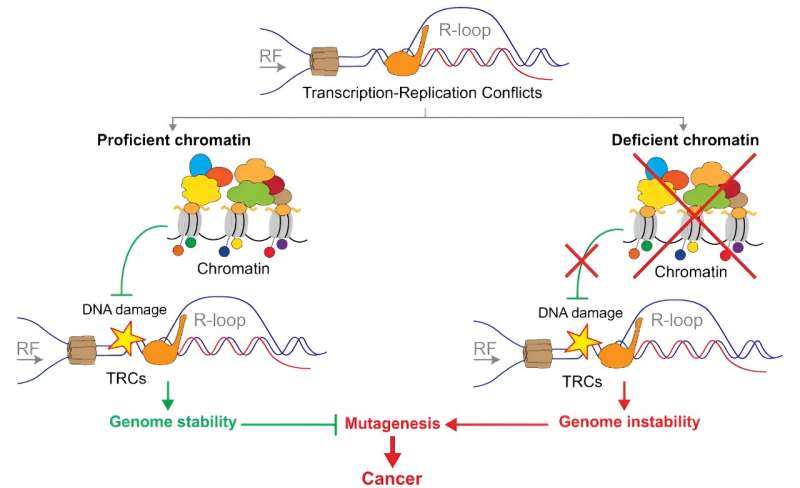
The study shows that chromatin and the factors regulating it prevent the formation of DNA–RNA hybrids, a source of genomic instability associated with cancer. These hybrids block replication, resulting in increased chromosomal cleavages and collisions between transcription and replication.
The research has shown that chromatin is a first barrier to protect the integrity of the genome. This has been observed in analyses of the silencing of various chromatin remodeling factors in tumor cell cultures.
In their study comparing their data with genome databases of tumor cells, they found that genome sites enriched in DNA–RNA hybrids match the sites with the highest frequency of mutations found in tumor cells. The work thus reveals for the first time that there is a direct association between DNA–RNA hybrids and cancer-associated mutations, suggesting that they are a risk factor in the development of tumors.
Aguilera’s lab has pioneered studies of the role of DNA–RNA hybrids in the development of genetic instability, and this new paper not only offers a better understanding of cellular control of hybrids and their regulation by epigenetic factors, but also points to the possibility that levels of DNA–RNA hybrids in cells could be used as a potential indicator of carcinogenic risk.
More information:
Aleix Bayona-Feliu et al, The chromatin network helps prevent cancer-associated mutagenesis at transcription-replication conflicts, Nature Communications (2023). DOI: 10.1038/s41467-023-42653-0
Journal information:
Nature Communications
Source: Read Full Article