
Large scale vaccination campaigns against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been implemented in several countries around the world. Although SARS-CoV-2 antibodies are not the only method that can be used to determine immunity against this virus, they act as important predictors of immunological protection. Unfortunately, the emergence of SARS-CoV-2 variants of concern (VoC) has challenged the effectiveness of current vaccines.
A new study available on the pre-print server medRxiv* determined the antibody response to SARS-CoV-2 and seasonal betacoronaviruses induced by BNT162b2-Comirnaty vaccination in healthcare workers with or without a prior history of the coronavirus disease 2019 (COVID-19).
 Study: Seasonal betacoronavirus antibodies expansion post BNT161b2 vaccination associates with reduced SARS-CoV-2 VoCs neutralization. Image Credit: Foxeel / Shutterstock.com
Study: Seasonal betacoronavirus antibodies expansion post BNT161b2 vaccination associates with reduced SARS-CoV-2 VoCs neutralization. Image Credit: Foxeel / Shutterstock.com
About the study
The current study involved 31 hospital health care workers (HCW), out of which thirteen were naïve to COVID-19 at vaccination. Notably, eighteen of the participants had a previous infection with SARS-CoV-2 during the first wave of the pandemic.
A plasmid was constructed for the expression of the SARS-CoV-2 spike protein variants using Lentiviral vector that also expressed luciferase. Two other assays including the neutralization assay and the immunoglobulin G (IgG) binding antibody Luciferase Immuno Precipitation System (LIPS) assay were also performed.
Decline in nAbs in vaccinated individuals with and without a history of COVID-19
Before receiving their vaccines, 6 of the 18 individuals with a prior history of COVID-19 did not have detectable neutralizing antibodies (nAbs) and IgGs that bound to the receptor-binding domain of the spike protein from the wildtype strain of SARS-CoV-2. Comparably, all vaccinated participants had nAbs that bound to both the wildtype SARS-CoV-2 strain, as well as both its RBD and S2 domains.
When evaluated against the Alpha and Beta VoCs, the nAbs and RBD IgGs in vaccinated individuals exhibited similar kinetics to that which was observed against the original strain of SARS-CoV-2. Notably, delayed and lower peak titers were observed in vaccinated individuals who did not present with detectable antibodies against SARS-CoV-2 at baseline.
Interestingly, nAb peak levels were found to increase according to the disease severity in individuals who had a history of COVID-19. However, regardless of their disease severity, there was a clear reduction in nAb peak values against the Beta VoC.
Taken together, the researchers found that vaccinated individuals with a history of COVID-19 frequently exhibit a rapid reduction below the detection limit of antibodies that are capable of also neutralizing VoCs.
BNT162b2 boosts antibodies against seasonal betacoronaviruses
The data also shows that BNT162b2 vaccination produces an early boost of antibodies to the S2 subunit of OC43 and HKU1 seasonal betacoronaviruses. This boost was reported in more than 50% of study participants who did not have a previous history of SARS-CoV-2 infection.
Notably, all individuals who had presented with SARS-CoV-2 antibodies at baseline had a significantly greater antibody response SARS-CoV-2 as compared to those against the seasonal betacoronaviruses. Additionally, a rapid increase in IgG against HKU1 ten days post-vaccination was associated with a faster reduction and loss of antibodies against SARS-CoV-2 especially with respect to the VoCs.
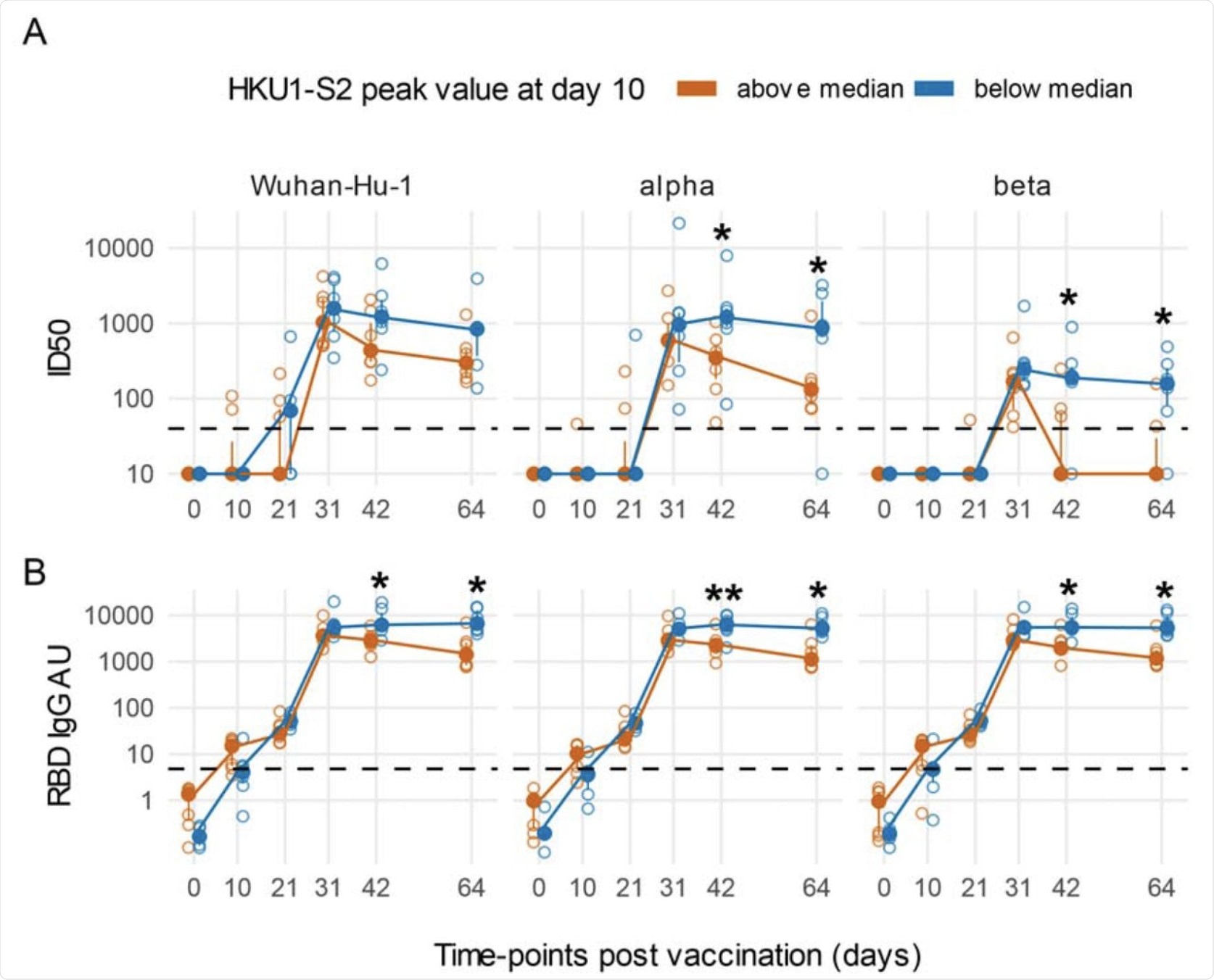 An early boost of HKU1 IgGs post-vaccination is associated with a rapidly decreasing Ab response against SARS-CoV-2 in COVID-19 naïve subjects. Line plots of Wuhan-Hu-1 and VoCs ID50 nAbs (A) and RBD IgG AU (B). Subjects naïve for COVID-19 were stratified as above or below the median of HKU1 S2 IgGs at day 10 post-vaccination. Filled circles and bars represent the median ± inter quartile range at each time-point, empty circles show each individual measurement. The horizontal dashed lines stand for the threshold for positivity. The asterisks indicate statistically significant differences in mean ID50 nAbs or RBD IgG AU at the corresponding time-points between subjects with or without an early boost of seasonal coronaviruses responses after vaccination (two-way repeated measures ANOVA, * p < 0.05, ** p< 0.01).
An early boost of HKU1 IgGs post-vaccination is associated with a rapidly decreasing Ab response against SARS-CoV-2 in COVID-19 naïve subjects. Line plots of Wuhan-Hu-1 and VoCs ID50 nAbs (A) and RBD IgG AU (B). Subjects naïve for COVID-19 were stratified as above or below the median of HKU1 S2 IgGs at day 10 post-vaccination. Filled circles and bars represent the median ± inter quartile range at each time-point, empty circles show each individual measurement. The horizontal dashed lines stand for the threshold for positivity. The asterisks indicate statistically significant differences in mean ID50 nAbs or RBD IgG AU at the corresponding time-points between subjects with or without an early boost of seasonal coronaviruses responses after vaccination (two-way repeated measures ANOVA, * p < 0.05, ** p< 0.01).
Conclusion
Although the current vaccines are found to be effective in reducing infection and disease severity of SARS-CoV-2, public health policies must adapt to the emerging VoCs. Further investigation regarding the choice of diagnostic tests, vaccine formulation, as well as vaccine deployment will help in the ongoing development of pan-coronavirus vaccines.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Dispinseri, S., Marzinotto, I., Brigatti, C., et al. (2021). Seasonal betacoronavirus antibodies expansion post BNT161b2 vaccination associates with reduced SARS-CoV-2 VoCs neutralization. medRxiv. doi:10.1101/2021.08.15.21262000. https://www.medrxiv.org/content/10.1101/2021.08.15.21262000v1
Posted in: Medical Science News | Medical Research News | Medical Condition News | Disease/Infection News | Healthcare News
Tags: Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Diagnostic, Health Care, Healthcare, Hospital, immunity, Immunoglobulin, Luciferase, Pandemic, Plasmid, Protein, Public Health, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article