
A new study performed in human lung airway cells is one of the first to show a potential link between exposure to organophosphate pesticides and increased susceptibility to COVID-19 infection. The findings could have implications for veterans, many of whom were exposed to organophosphate pesticides during wartime.
Exposure to organophosphate pesticides is thought to be one of the possible causes of Gulf War Illness, a cluster of medically unexplained chronic symptoms that can include fatigue, headaches, joint pain, indigestion, insomnia, dizziness, respiratory disorders and memory problems. More than 25% of Gulf War veterans are estimated to experience this condition.
“We have identified a basic mechanism linked with inflammation that could increase susceptibility to COVID-19 infection among people exposed to organophosphates,” said Saurabh Chatterjee, Ph.D., from the University of South Carolina and a research health specialist at the Columbia VA Medical Center and leader of the research team. “This mechanism could also increase risk for people with metabolic diseases and cancer because they tend to exhibit the same type of inflammation.”
Ayan Mondal, Ph.D., a postdoctoral fellow in Chatterjee’s lab, will present the research at the American Society for Biochemistry and Molecular Biology annual meeting during the virtual Experimental Biology (EB) 2021 meeting, to be held April 27-30.
“The reason why COVID-19 causes a severe form of disease leading to hospitalization and high rates of mortality in a small segment of society is unclear,” said Prakash Nagarkatti, co-author of the study and vice president for research at the University of South Carolina. “This work sheds new light on exposure to pesticides and potential susceptibility to COVID-19 through altered immune response.”
In previous work, the researchers found increased interleukin 6 (IL-6) levels in samples from veterans and a mouse model of Gulf War Illness. The body produces these proinflammatory proteins to help fight infections and respond to tissue injuries. However, continual production of IL-6 can lead to chronic inflammation and has been shown to decrease the immune system’s response to viruses.
In the new study, the researchers wanted to find out whether exposure to the organophosphate pesticide chlorpyriphos and increased levels of IL-6 could increase risk of SARS-CoV-2 infection. For six hours, they exposed human lung airway epithelial cells to either IL-6 or chlorpyriphos or to both in combination. Another group of cells received no exposure to serve as a control.
The researchers then treated the cells with the spike proteins that cover the outside of SARS-CoV-2, the virus that causes COVID-19. During infection, spike proteins bind with angiotensin converting enzyme 2 (ACE2) receptors our cells, starting a process that allows the virus to release its genetic material into the healthy cell. The researchers found that cells exposed to IL-6 and the pesticide exhibited increased apoptosis—or controlled cell death—when the SARS-CoV-2 spike protein was present.
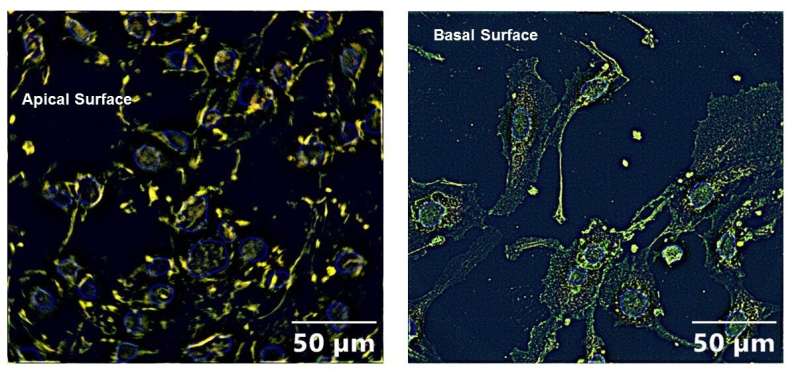
The cells exposed to both the pesticide and IL-6 also had significantly more ACE2 expression on the apical cell surface compared to cells that were unexposed or exposed to the pesticide alone. The apical membrane of airway cells faces the interior of the airway while the basolateral membrane touches the surrounding tissues. Increased ACE2 receptor expression on the apical surface means more virus will attach to the cells.
“To our knowledge, this is the first study demonstrating that the ACE2 receptor translates from the basolateral cell membrane to the apical cell upon co-exposure to organophosphate and IL-6,” said Chatterjee. “Since people with obesity, type 2 diabetes or cancer also have high circulatory IL-6 levels, we think people with these conditions will also have increased susceptibility to SARS-CoV-2 infection because of increased translocation of ACE2 receptor to the apical cell surface.”
Source: Read Full Article