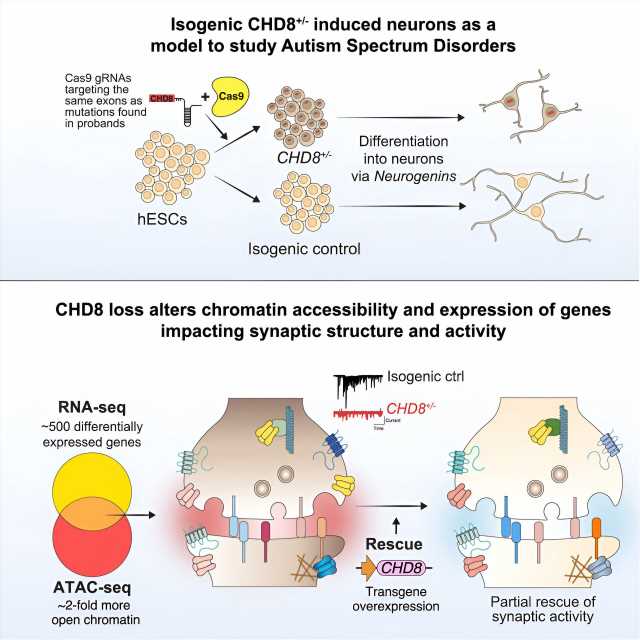
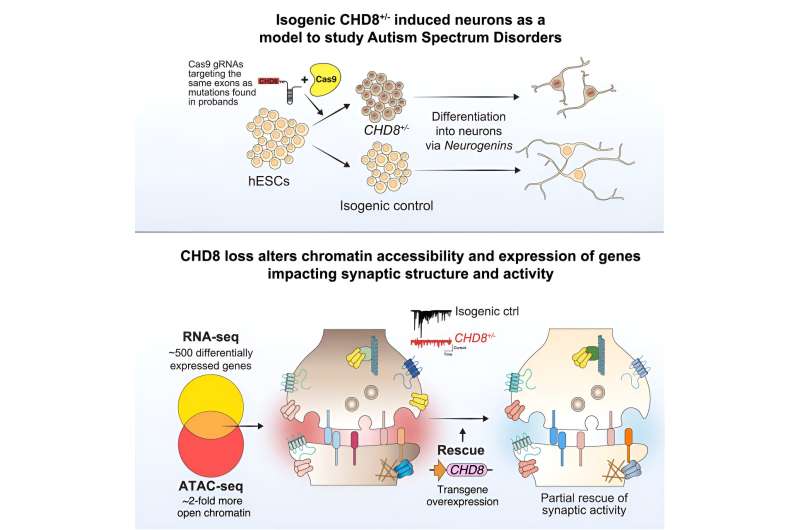
Researchers have used CRISPR gene editing, stem cells and human neurons to study the impact of a gene that is commonly mutated in autism. This new study, published today in The American Journal of Human Genetics, ties mutations in the gene CHD8 with a broad spectrum of molecular and cellular defects in human cortical neurons.
Autism is a highly heritable disorder with a recent increase in incidence—approximately 1 in 40 children in the US are diagnosed with autism. Over the past decade, sequencing studies have found many genes associated with autism but it has been challenging to understand how mutations in certain genes drive complex changes in brain activity and function.
The study was led by researchers in Dr. Neville Sanjana’s lab at the New York Genome Center and New York University (NYU) and in Dr. Jen Pan’s lab at the Broad Institute and includes co-authors from the Massachusetts Institute of Technology (MIT), Cold Spring Harbor Laboratory, ETH Zurich and McGill University. The team developed an integrated approach to understand how mutations in the CHD8 gene alter genome regulation, gene expression, neuron function, and are tied to other key genes that play a role in autism.
For more than a decade, it has been known that individuals with mutations in the CHD8 gene tend to have many similar ailments, such as autism, an abnormally large head size, digestive issues and difficulty sleeping. The CHD8 gene is a regulator of proteins called chromatin that surround the DNA but it is unclear how this particular gene might relate to major alterations in neural development and, in turn, result in autism.
The research team identified numerous changes in physical state of DNA, which makes the genome more accessible to regulators of gene expression, and, in turn, drives aberrant expression of hundreds of genes. These molecular defects resulted in clear functional changes in neurons that carry the CHD8 mutation. These neurons are much less talkative: They are activated less often and send fewer messages across their synapses.
The study authors initially observed these changes using human cortical neurons differentiated from stem cells where CRISPR was used to insert a CHD8 mutation. These findings were further bolstered by similar reductions in neuron and synapse activity when examining neurons from mice with a CHD8 mutation.
These substantial defects in neuron function were circumvented when extra CHD8 was added to the cell using a gene therapy approach. In this case, extra copies of a healthy CHD8 gene without any mutation were added using a viral vector. Upon differentiation, the team found that the neurons rescued by the treatment returned to a normal rate of activity and synaptic communication, indicating that this gene therapy approach may be sufficient to restore function.
Lastly, when examining disrupted genes, the authors found that the CHD8 mutation seemed to specifically alter other genes that have been implicated in autism or intellectual disability, but not genes associated with unrelated disorders like cardiovascular disease. This suggest that CHD8 might influence selectively those genes that tend to be involved in neurodevelopmental disorders, providing an explanation for some of the particular characteristics of individuals carrying a CHD8 mutation.
More information:
Xi Shi et al, Heterozygous deletion of the autism-associated gene CHD8 impairs synaptic function through widespread changes in gene expression and chromatin compaction, The American Journal of Human Genetics (2023). DOI: 10.1016/j.ajhg.2023.09.004
Journal information:
American Journal of Human Genetics
Source: Read Full Article