
In their continuing work to limit the impact of COVID-19, Emory University researchers have, for the first time in nonhuman primates, studied how modulating the signaling of type 1 Interferon (IFN-I), one of the body’s initial defenses against infection, impacts SARS-CoV-2 viral replication and disease progression. The findings, which are reported in Science Immunology, demonstrate a critical role of IFN-I in SARS-CoV-2 infection and could help in the development of new treatment strategies for COVID-19.
Senior author Mirko Paiardini, Ph.D., led the study that showed while early antiviral IFN-I responses are crucial for restraining SARS-CoV-2 replication, excessive IFN-I signaling contributes to hyperinflammation in rhesus macaques. This animal species is a critical model for studying mild/moderate COVID-19 and is helping researchers discover information to limit the virus’ impact, which the WHO COVID-19 Dashboard lists as nearly 767 million cases and nearly 7 million deaths.
“Our finding reinforces how critical it is to prevent or quickly treat excessive inflammation, which is considered the main cause of severe COVID-19,” Paiardini says. He is division chief of Microbiology and Immunology at the Emory National Primate Research Center (EPC), professor of Pathology and Laboratory Medicine at Emory University School of Medicine and at the Emory Vaccine Center, co-director at the Emory Center for AIDS Research and the lead principal investigator of the ERASE-HIV Martin Delaney Collaboratory for HIV Cure Research.
Working in close collaboration with Paiardini on this study were Steve Bosinger, Ph.D., co-first authors Elise Viox, Timothy Hoang, Ph.D., and Amit Upadhyay, Ph.D., and R. Paul Johnson, MD. Bosinger is director of the EPC Nonhuman Primate Genomics Core and assistant professor in the Emory School of Medicine Department of Pathology & Lab Medicine. Johnson is director of the EPC
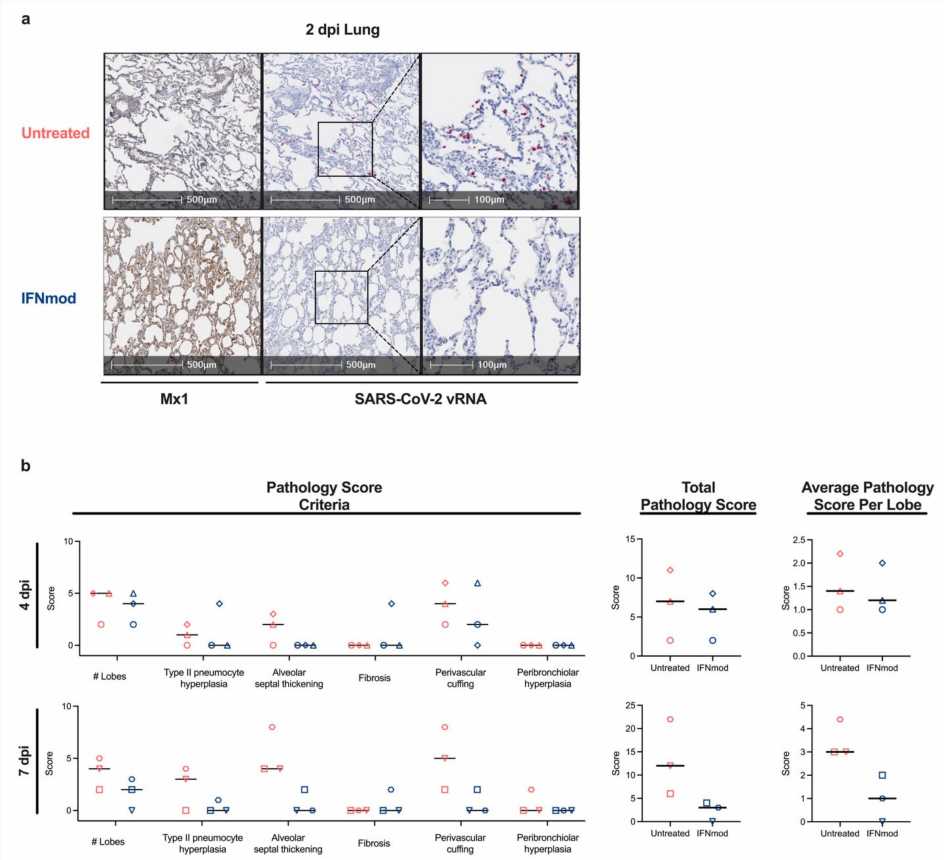
The researchers used a mutated version of interferon (IFNmod) to modulate IFN-I signaling in rhesus macaques before and during acute SARS-CoV-2 infection. Their results showed IFNmod treatment weakened antiviral and inflammatory gene expression and led to lower levels of inflammatory cytokines, substances that have an effect on other cells, in the lower airways and reduced lung pathology.
“We were also surprised to find IFNmod treatment had a profound effect on SARS-CoV-2 viral loads, with a 3,000-fold reduction in viral loads in the lower airways of treated animals,” says Viox.
The research team dedicates this scientific publication in loving memory to co-first author Hoang whose commitment to this project and to scientific discovery were crucial in propelling this study forward. Hoang is remembered for his intelligence, drive and love for science, and all of the lives he touched during his short but impactful career.
The research reported in this release is supported in part by the National Institutes of Health, including Office of the Director, Emory University, Fast Grants, a private foundation, the German Research Foundation and the German Federal Ministry for Research and Education.
More information:
Elise G. Viox et al, Modulation of type I interferon responses potently inhibits SARS-CoV-2 replication and inflammation in rhesus macaques, Science Immunology (2023). DOI: 10.1126/sciimmunol.adg0033
Journal information:
Science Immunology
Source: Read Full Article