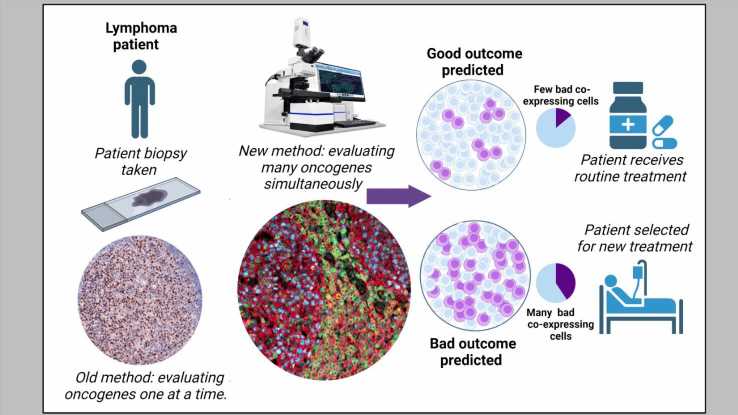
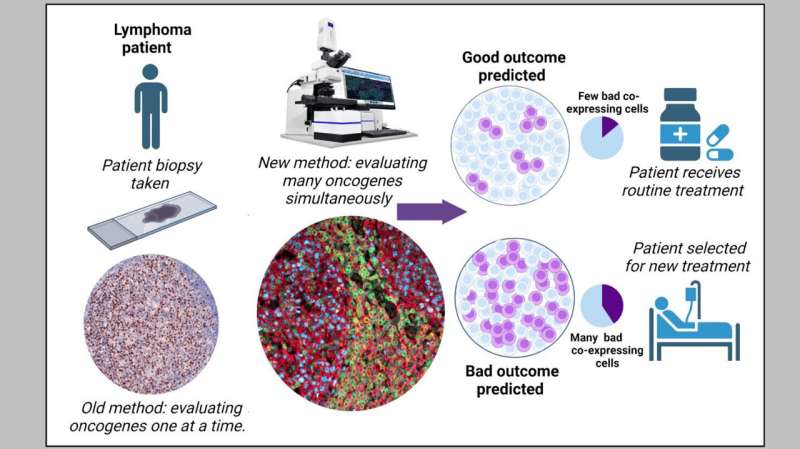
A team of researchers from the Cancer Science Institute of Singapore (CSI Singapore) at the National University of Singapore (NUS), led by Assistant Professor Anand Jeyasekharan, has discovered a unique combination of oncogenes that could predict treatment resistance, and hence unfavorable outcomes, of patients with diffuse large B cell lymphoma (DLBCL), the most common type of blood cancer in Singapore and globally.
This unique oncogenic combination, serving as an indicator of treatment resistance, can be detected through state-of-the-art technology. The researchers, however, went a step further to develop a simple mathematical formula that can predict the fraction of cells with this unique unfavorable combination from information obtained through established diagnostic methods—paving the way for this oncogene indicator to be used in routine clinical practice. Their findings were published in Cancer Discovery on 4 May 2023.
Understanding the co-existence of oncogenes in blood cancer
Oncogenes play a crucial role in cancer development by directing the production of “bad” oncoproteins that promote the growth and survival of cancer, while influencing treatment outcomes. However, cancers frequently express multiple such oncogenes, and not all cancer cells express every oncogene. As oncogenes are typically studied one at a time, very little is known about how the “co-existence” of oncogenes in subsets of cancer cells impacts the survival of cancer patients.
To fill this gap in knowledge, the research team set out to determine whether, and how, oncogenes work together to resist treatment, and they studied the phenomenon in the setting of DLBCL.
Current clinical practice routinely employs immunohistochemistry to measure the three specific oncogenes—MYC, BCL2, and BCL6—to identify high-risk cases of DLBCL. However, immunohistochemistry cannot detect these three oncogenes simultaneously and, therefore, is unable to identify subgroups of cells with specific oncogenic combinations.
To overcome this challenge, the team used a state-of-the-art imaging technology—termed multispectral microscopy with quantitative immunofluorescence—to stain, image, and quantify these oncogenes simultaneously in a large number of samples from DLBCL patients.
Unfavorable combination
The researchers discovered that patients with a high percentage of cells that are positive for MYC and BCL2, but negative for BCL6, have low survival rates. The presence of these types of cells results in the lowest survival rates compared to all other cellular combinations of the three oncogenes, including those that are positive for all three.
Based on this significant finding, the team concluded that BCL6 plays a “protective” role by “restraining” otherwise aggressive cells that have both MYC and BCL2 oncogenes. They subsequently identified potential mechanisms for why BCL6 is “protective” of cells with high MYC and BCL2.
The team also developed a mathematical formula that uses immunohistochemistry data to “predict” the proportion of cells with this specific harmful oncogenic combination. This is currently being further investigated through international collaborations led by the team at CSI Singapore.
Explaining the significance of the team’s research, Asst Prof Jeyasekharan, corresponding author of the study and Principal Investigator at CSI Singapore, said, “Our findings are highly relevant for the clinical management of DLBCL. Furthermore, they could potentially suggest a paradigm that is applicable to other cancer types as well, in that simultaneous detection of oncogenes is vital to understand their clinical relevance.”
More information:
Michal Marek Hoppe et al, Patterns of Oncogene Coexpression at Single-Cell Resolution Influence Survival in Lymphoma, Cancer Discovery (2023). DOI: 10.1158/2159-8290.CD-22-0998
Journal information:
Cancer Discovery
Source: Read Full Article