
In a recent study published on the medRxiv* preprint server, researchers performed two parallel population-based studies using the National Immunization Management System (NIMS) data in England to investigate the possible relationship between COVID-19 vaccination and the incidences of Guillain-Barre syndrome (GBS).

Study: COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database. Image Credit: Verin / Shutterstock.com
While assessing the neurological impact of SARS-CoV-2 infection, brachial neuritis, facial palsy, and Guillain-Barre syndrome (GBS) have been the subjects of particular interest. However, an association of GBS with COVID-19 vaccination remains unknown.
During the swine flu vaccination campaign in the United States, GBS became an adverse event of special interest (AESI) related to vaccination. From January 2020 onwards, the COVID-19 vaccination campaigns in the United Kingdom triggered extensive monitoring with GBS as an AESI.
About the study
In the current study, the researchers retrospectively interrogated the database of patients hospitalized with GBS in England, Scotland, and Northern Ireland, along with the NIMS COVID-19 vaccinations data. They also characterized a large surveillance dataset of the incident U.K. GBS cases after COVID-19 vaccination and before vaccination during the same period, wherein they recorded the timing of onset after COVID-19 vaccination.
Study findings
A total of 996 GBS cases were reported to the National Immunoglobulin Database (NID) from January to October 2021. The number of GBS cases in January 2021 was significantly lower, continuing the trend of lower GBS rates of the years 2016-2020.
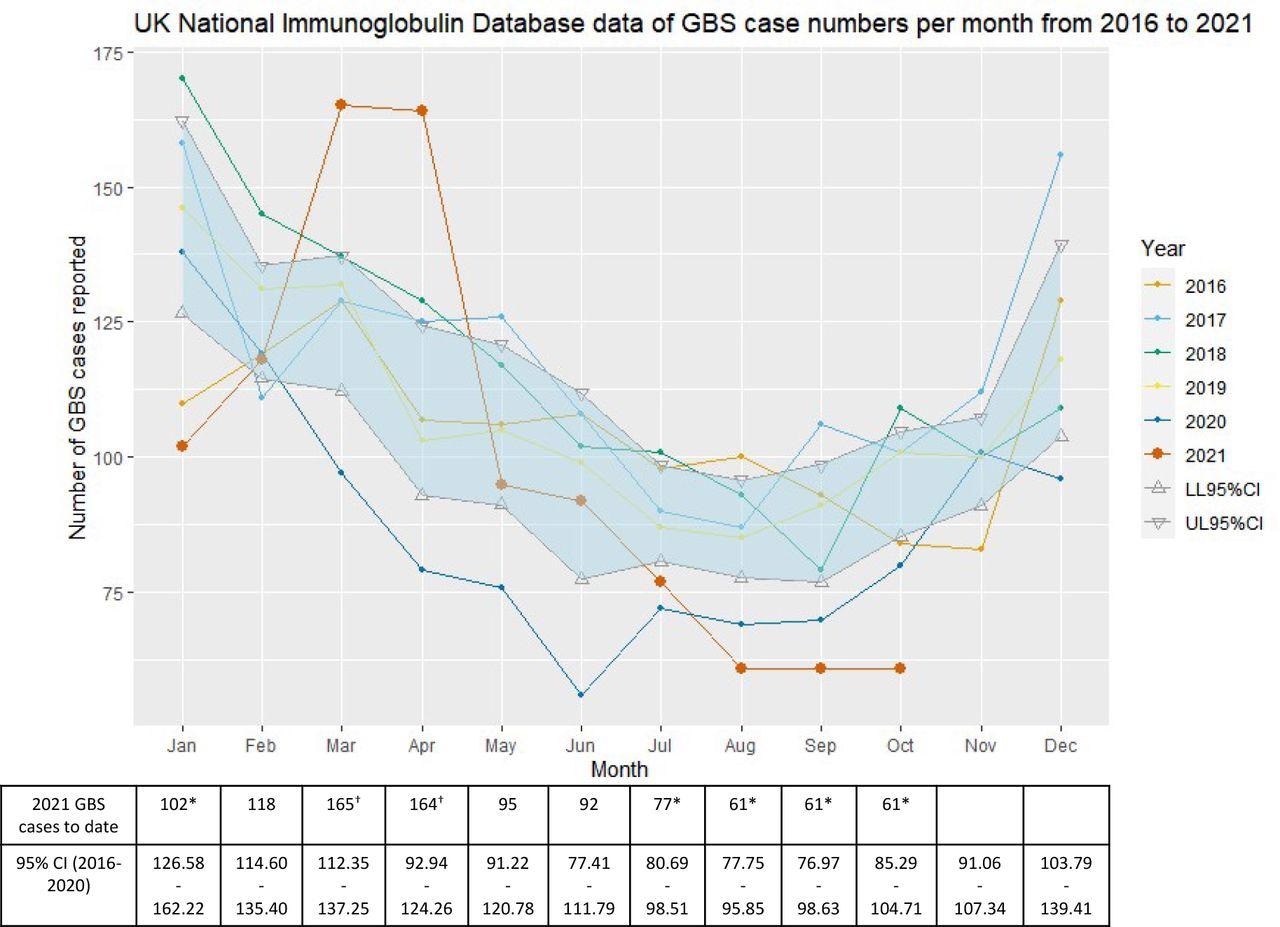
However, in March and April 2021, England, Scotland, and Northern Ireland experienced a notable monthly increase in GBS cases. Importantly, GBS rates fell again in the normal range of the 2016-2020 average from July to October 2021.
The U.K. COVID-19 vaccination program began on December 8, 2020, with the Pfizer-BioNTech BNT162b2 vaccine, followed by the AstraZeneca ChAdOx1 nCoV-19 in January 2021, and subsequently by the Moderna mRNA-1273 vaccine. By February 2021, 50% of adults over the age of 50 had taken their first vaccination.
While.8 cases of GBS were reported per million first doses of the ChAdOx1 nCoV-19 vaccine, GBS cases associated with the first dose of the BNT162b2 vaccine were insignificant. This data suggests that more GBS cases were associated with the first dose of ChAdOx1 nCoV-19 COVID-19 vaccination and occurred within the first 42 days following immunization.
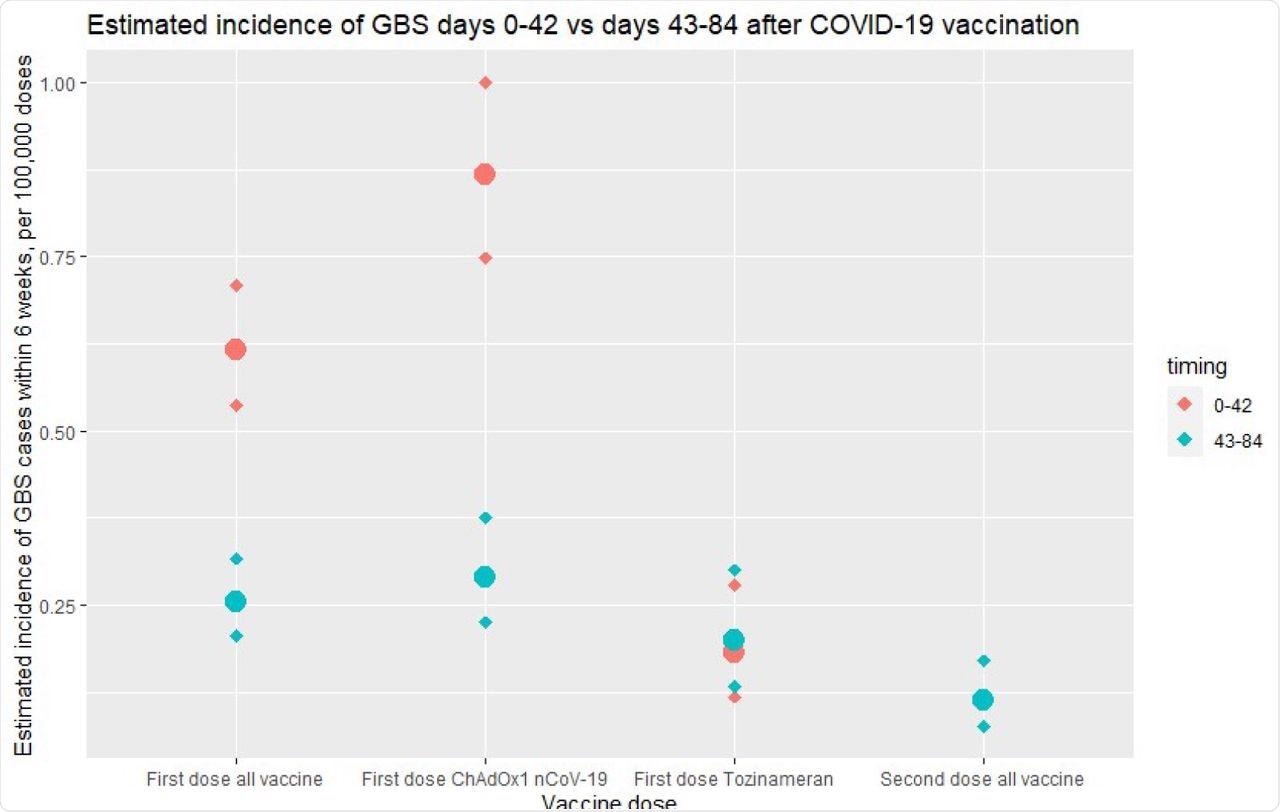
Excess risk in first 42 days following vaccination in England. Estimated incidence of GBS cases within 6 weeks (per 100,000 vaccine doses), comparing reports of GBS cases 0-42 days (red) and 43-84 (blue) for first-dose vaccines (all vaccines, ChAdOx1 nCoV-19, tozinameran) and second-dose vaccines. Diamonds represent upper and lower limits of 95% confidence intervals. An excess of GBS cases is noted in the first 42 days following first-dose vaccination, accounted for by the ChAdOx1 nCoV-19 vaccine.
All GBS cases reported to the NID did not have a vaccination record. The first GBS case within six weeks after a COVID-19 vaccination was reported in January 2021.
A total of 198 GBS cases occurred within six weeks at the rate of 0.618 cases per 100,000 vaccinations. Of these 198 cases, 176 cases occurred after the first dose of the ChAdOx1 nCoV-19 vaccine at a rate of 0.868 per 100,000, whereas 21 cases were reported after patients had received the first dose of the BNT162b2 vaccine at a rate of 0.183 per 100,000.
A total of 32.1 million first dose vaccinations were recorded during the study period, of which included 20.3 million ChAdOx1nCoV-19, 11.5 million BNT162b2, and 0.3 million mRNA1273 doses. Only one GBS case occurred within six weeks of mRNA-1273 vaccination.
Within six weeks of any second dose, 23 GBS cases occurred. The GBS incidence after the first vaccination was highest in males receiving the ChAdOx1 nCoV-19 vaccine at a rate of 1.069 per 100,000 doses. It remains unclear why more males were affected than females.
Of the total 121 GBS cases that were reported by the British Peripheral Nerve Society (BPNS) and the Association of British Neurologists (ABN) network between January and November 2021, 90% occurred between January and April 2021 within six weeks of vaccination and only 35% of cases occurred from May 2021 onwards. The median age of these GBS patients was 59 years, of which 59% were male. Moreover, 42 patients reported facial weakness associated with other GBS findings and only one patient had a recurrent GBS-like illness following second-dose vaccination.
Of the 121 GBS cases, 106 or 87.3% of patients had GBS before receiving COVID-19 vaccination, with 66.1% having taken the first dose of the vaccine within 42 days of GBS onset. In comparison with the linked NID/NIMS dataset, 198 of the 659 GBS cases in England were reported within 42 days of vaccination, thereby indicating the reporting bias of the dataset towards vaccine-associated GBS.
Conclusions
The current study demonstrates an association between the first dose of the ChAdOx1 nCoV-19 vaccine and GBS, which accounts for an estimated incidence of 5.8 GBS cases per million doses. While the reason for this association remains unclear, the risk remains almost similar to prior vaccine-associated GBS and the benefits of vaccination outshine the risk. Further research is required to confirm the study's observations, determine GBS-related causalities, and investigate the effects of other COVID-19 vaccines used globally.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Keh, R. Y. S., Cavanagh, S., Foster, M., et al. (2021). COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database. medRxiv. doi:10.1101/2021.12.14.21267418. https://www.medrxiv.org/content/10.1101/2021.12.14.21267418v1
Posted in: Medical Research News | Medical Condition News | Disease/Infection News | Pharmaceutical News
Tags: Coronavirus Disease COVID-19, Facial Palsy, Flu, Guillain-Barré Syndrome, Immunization, Immunoglobulin, Nerve, Research, SARS, SARS-CoV-2, Swine Flu, Syndrome, Vaccine

Written by
Neha Mathur
Neha Mathur has a Master’s degree in Biotechnology and extensive experience in digital marketing. She is passionate about reading and music. When she is not working, Neha likes to cook and travel.
Source: Read Full Article