
As our bodies grow throughout childhood and puberty, our final height and the shape of our skeleton are largely determined by growth plates—areas of new growth at both ends of the long bones, such as those in the arms, legs, hands and feet. These areas expand, and the cartilage they are made of gradually hardens into solid tissue, adding length and width to the bones. Researchers at the Weizmann Institute of Science have developed a method that makes it possible to examine, for the first time, the cellular makeup of growth plates in three dimensions. The method has enabled new insights into the growth of healthy bone and the ways that this growth may be disrupted in dwarfism.
Nearly a century ago, scientists studying bone growth had already talked about the need to focus on the changes in cartilage cells at both ends of long bones. These cells create a sort of template that is later replaced by mineralized bone tissue, and deviation from the norm in these cells can presage future trouble. “If the cartilage is abnormal, you end up with abnormal bone—it may be too short, too long or deformed,” explains Prof. Elazar Zelzer of the Molecular Genetics Department, who headed the research team.
Yet no effective way of tracking the processes cartilage cells undergo within the growth plate had been available. The methods for examining these cells provided images in two dimensions, giving researchers only a partial picture of the cells’ volume and spatial arrangement. Moreover, the high-resolution methods yielded detailed images, but only of small portions of the growth plate, thus missing the full picture.
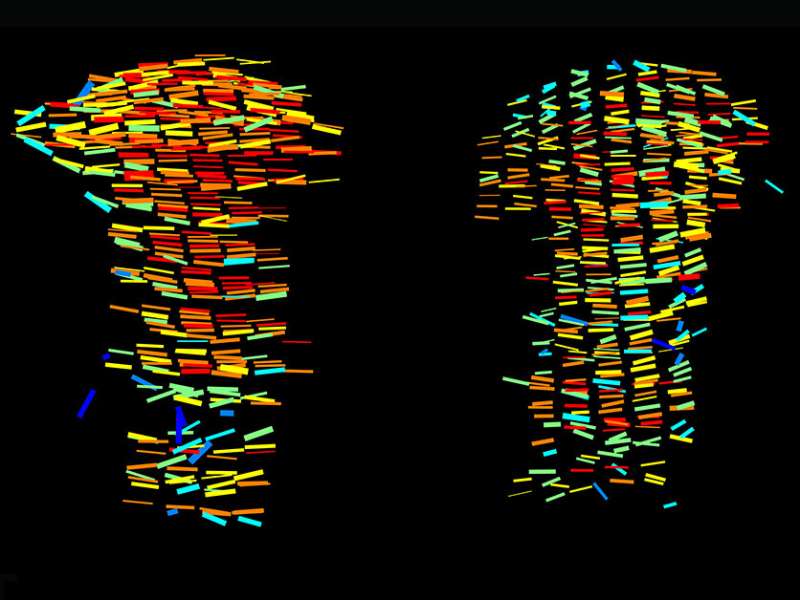
Doctoral student Sarah Rubin in Zelzer’s lab led the new study, in which she and the other scientists created a multistep method, 3D MAPs, for exploring hundreds of thousands of cartilage cells in three dimensions. First, the scientists treat growth plate tissue with chemicals that make it transparent so they can obtain images using a technique called light-sheet microscopy. They then slice up the images into segments and apply a series of algorithms that characterize the cells in each cross section. Finally, they map the cells back onto the full image, obtaining a high-resolution 3D map of the entire growth plate that reveals the shape and size of all the cartilage cells, as well as their spatial orientation and organization.
The method shows, in unprecedented detail, what happens to cells within the growth plate, and it has already overturned a previous assumption about bone growth. It was known that the cartilage cells that are born at both tips of the bone change continuously, so that the “oldest” ones—that is, those closest to the middle of the bone—are in the final stage of transformation. In previous studies, these “oldest” cells appeared nearly ten times larger than those at the preceding stage. But when the scientists used 3D MAPs to observe the growth plates of mice, they discovered that this supposed jump in size had in fact been an illusion resulting from the distortions of two-dimensional imaging. Rather, the increase in cell size is relatively gradual, with much of it taking place at the next-to-final stage of the cells’ transformation.
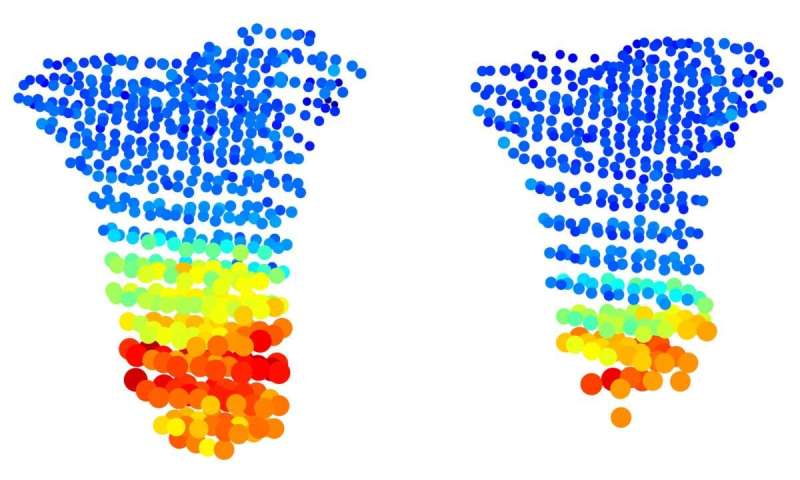
With 3D MAPs the scientists have also managed to solve two long-standing riddles concerning a form of dwarfism called Grebe syndrome, which is caused by a mutation in the Gdf5 gene. This gene was known to regulate the formation of joints, so it was not clear why its mutated form causes people to be born with severely shortened and deformed limbs. Nor was it clear why the growth plate that gave rise to such abnormal bones in people with the syndrome had appeared to be completely normal in earlier studies.
When the scientists applied 3D MAPs to the bones of young transgenic mice having the Gdf5 mutation, the method revealed an entire range of abnormalities in the growth plate that had not been picked up by previous studies. These abnormalities suggested that in addition to its role in joint formation, the Gdf5 gene regulates the transformation of cartilage cells within the growth plate. When this gene was mutated, cartilage cells failed to increase in size at the right stages, their shape was abnormal and they were not properly oriented.
“Think about tightly packed marbles—if you alter their shape, you won’t be able to pack them in a box in the same way as before,” says Rubin. “Similarly, altered cartilage cells cannot be arranged correctly inside the growth plate, and this prevents the bone from elongating normally.”
The new method provides a means of further exploring the cellular mechanisms operating within the growth plate in bone that is healthy, as well as in that affected by various abnormalities. It may also be used to study external influences on the bone—for example, how its growth is affected by interaction with muscles.
Zelzer says that “the growth plate can be viewed as an engine that drives the elongation of bone. We’ve now lifted the hood to get a close look at how this engine works and what happens when it breaks down.”
Source: Read Full Article